The Activation Energy Is Best Described by
There is no relationship between activation energy and rate of a reaction. Activation energy can be described as the a.
Activation Energy And Temperature Dependence Boundless Chemistry
The energy required for a reaction to proceed by breaking bonds d.
. Activation energy in chemistry the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo a chemical transformation or physical transport. So I think its C. This can be understood by turning once again to the reaction between ClNO 2 and NO.
The temperature at which the reaction proceeds most quickly. 1 review of The Inner Fuel - Kundalini Activation Process I met Eugenia in a yoga class and she explained what kundalini was never in my life I have ever heard of such a word nor what it meant. A type of exergonic reaction b.
B the minimum kinetic energy that particles must possess for a chemical reaction to occur. Because activation energy is the amount of energy required for a chemical reaction to take place. D the energy required to separate ions in a crystalline solid.
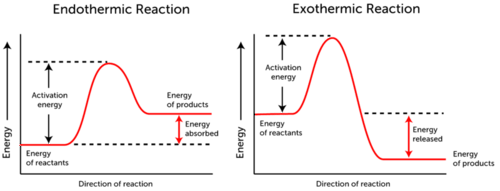
Specifically the higher the activation energy the slower the chemical reaction will be. The minimum amount of additional energy needed by a reacting molecule to get transformed into the product is termed activation energy. Energy of the activated complex.
The source of the activation energy needed to push reactions forward is typically heat energy from the surroundings. The difference in free energy between the products and the transition state. Activation energy is a concept used in chemistry that was introduced by the scientist from Sweden named Svante Arrhenius in 1889.
The energy level of the reactants. C the energy of motion. Heat energy from the atmosphere is usually the source of the activation energy used to drive reactions forward.
Energy difference between the reactants and the products. A type of endergonic reaction c. Activation energy is the minimum amount of energy needed to start a chemical reaction.
In simple terms it is the amount of energy that needs to be supplied in order for a chemical reaction to proceed. How do temperature and activation energy relate to. Activation energy is the amount of energy required to reach the transition state.
4Products have higher chemical potential energy than reactants. Which of the following best describes the activation energy of a chemical reaction. 17Which form of inhibition involves a molecule binding in the same position of the enzymes active site as.
Rank the grades of coal by their relative desirabilities starting with mos desirable at the top. The energy level of the products. The difference in free energy between.
Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction. Energy difference between the reactants and the activated complex. A numerical description of the amount of energy needed by colliding reactant molecules in order to form.
Which statement best describes the relationship between activation energy and rate of reaction. 3White phosphorus has a lower activation energy than red phosphorus. Increasing the activation energy can increase the rate of a reaction.
Activation energy is best described as ___ the energy required to initiate a chemical reaction. 1energy input needed to break bonds of reactants. The activation energy of a chemical reaction is closely related to its rate.
So less than 40KJmol of activation energy is safer. However the way she described it I decided to be open enough to try it. The activation energy in the Arrhenius equation can best be described as asked Jun 26 2017 in Chemistry by maju88 a.
Activation energy is the minimum amount of energy required by chemical reactants to undergo a chemical reaction. The energy of activation below 30KJmol 40KJmol is known as the minimal energy of activation. For cellular reactions to occur fast enough over short time scales their activation energies are lowered by molecules called catalysts.
One association occurs between the energy of activation and the reaction rate. The Activation Energy of Chemical Reactions. I want to say that this ladys soul is so warming and welcoming that I wasnt as nervous as I thought Id be only.
The difference in energy between reactants and the maximum energy. Activation energy is also defined as the least possible energy required to initiate a chemical reaction. The difference in energy between reactants and products.
Reducing the activation energy can increase the rate of a reaction. The catalysts needed to raise a reactions rate. This is the starting energy before we go on the reaction.
Activation energy can best be described as. 2energy stored in chemical bonds. 1Which phrase defines activation energy1 point energy output when product bonds are formed energy input needed to break bonds of reactants energy stored in chemical bonds energy required to break a chemical bond 2Which phrase defines chemical potential energy1 point energy stored in chemical bonds energy input needed to break reactant bonds.
The maximum energy level of the reaction. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier. 16Which best describes the energy of activation.
The difference in free energy between the reactants and products of the reaction D.

Kinetics Why Is Activation Energy Drawn In A Potential Energy Diagram In Reactions Chemistry Stack Exchange

Is Activation Energy Negative Or Positive

Comments
Post a Comment